Gabby (Nielk) and Russell (Firestormm) continue our summary of the FDA Workshop with a look at the first part of Day Two – a discussion entitled “Innovation, Expedited Pathways, and Regulatory Considerations”
 The FDA Drug Development Workshop for Chronic Fatigue Syndrome (CFS) and Myalgic Encephalomyelitis (ME) took place on the 25th and 26th of April 2013. It was well attended by patients, advocates and doctors, together with representatives from the CDC and pharmaceutical companies.
The FDA Drug Development Workshop for Chronic Fatigue Syndrome (CFS) and Myalgic Encephalomyelitis (ME) took place on the 25th and 26th of April 2013. It was well attended by patients, advocates and doctors, together with representatives from the CDC and pharmaceutical companies.
The workshop was also broadcast live over the internet, enabling many more people to follow what happened without being there in person. This meeting was an opportunity for us to be heard, and continues to be an opportunity as comments can still be added to the docket until August 2nd 2013.
The FDA have clarified that they will accept video submissions in the form of links to YouTube videos – allowing those patients who were unable to attend the workshop to have their say. You can submit written comment on the docket (2,000 word limit), attach a Word or PDF document, or include a link to a YouTube video.
This is the third in a series of articles about the Workshop which attempt to summarize 85,000 words of talk over two days into only a few thousand words. Part One can be found here and Part Two, here. This article covers the content of the fifth video from the workshop.
Day Two, Panel 1 – Innovation, Expedited Pathways and Regulatory Considerations
[Video Five] [Phoenix Rising Transcript]
PANEL PRESENTATIONS:
1. Drug Innovation and De-risking Drug Discovery
 Dr Bernard Munos, MS MBA, Founder, InnoThink Centre for Research in Biomedical Innovation [Timestamp: 4:55-31:35]
Dr Bernard Munos, MS MBA, Founder, InnoThink Centre for Research in Biomedical Innovation [Timestamp: 4:55-31:35]
Dr Munos’ motivation came from a concern that the culture in the drug industry had become dominated by process at the expense of innovation and creativity, and that this was hampering progress.
“This was an industry that spent tens of billions of dollars on research every year and yet no one had a clue about where innovation comes from and how to get more of it and I thought that was mismanagement on a grand scale…”
His role – indeed his career for the past 10 years – has been to better understand what produces innovation and to try and help reverse this trend. If this can be helped it will result in a significant improvement in research advances and we will see the more efficient production of new drugs; something that would be of great benefit to the CFS community.
But what can our community do to help stimulate interest from the drug industry and from scientists in general? How might we better attract their interest and engagement? In short, what more can we do to help them to help us?
Dr Munos considered our disease and the problems currently associated with it. He proposed that the reason there is a lack of advancement in the field of CFS research compared to others like AIDS and HIV, is because ours is a complex disease, poorly understood and with multiple theories relating to mechanism and cause. It is thus a confusing and ill defined illness, and this doesn’t attract the necessary attention from scientists or the FDA.
“The problems associated with innovation in CFS are compounded by the fact this is a complex disease that is often mistaken for other diseases, its borders are fuzzy, and we have got multiple etiologies; so it’s not a simple thing to deal with.”
Similarly, there is a lack of interest from the pharmaceutical industry because CFS does not have an established scientific discipline like Cancer or Diabetes. Another problem is that treatments so far have targeted the symptoms and not any underlying disease because these processes have not been satisfactorily defined.
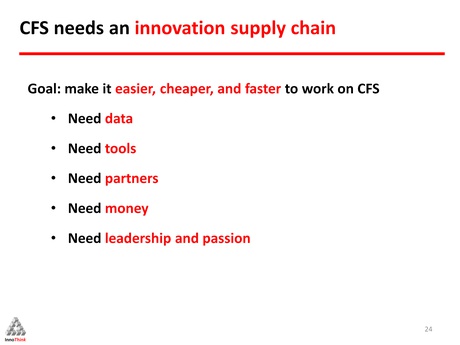
In addition, and importantly, there is a lack of good data for CFS. He believes that if the CFS community wants more scientists to work on their disease, then the community itself should make it worth their while and in order to do that, it needs to work out how to make it easier, cheaper and faster for them to do so.
“We need more and better data. We need tools to go along with the data. We need partners, we need money and lastly, we need leadership and passion. I should stress we have quite a bit of that already, but we need to, [perhaps], connect it a little bit better than it is connected today.”
Our community already have the patient registry in America for CFS, but he felt it would be better if this data could be collected from sources across the world. This would lead to a much broader and more variable array of information, producing a better understanding of the disease differentials. Note: this is something that might be achieved by the OMI-MERIT initiative and their International Neuro Registry and Biobank.
“If you think about the scientific revolutions in history, they’ve been driven by one thing: the availability of data. From Copernicus to quantum mechanics: it is data that drives innovation.”
One way to collect data direct from patients is with the use of bio-sensors in say, a cell-phone or through use of a ‘Fit Bit‘. This latter device easily monitors all activity undertaken, in real-time, which can then be uploaded to a central facility at very little relative cost.
He also commented that collecting genomic data from patients could prove to be extremely useful, especially as the costs today have become far less prohibitive, and are expected to fall even further in coming years.
The FDA will love the idea of high quality data collection and, since industry is unlikely to undertake such a vital step (for the reasons outlined above); he believes the opportunity must be seized by the patient community.
“The tools that we need are the basic tools of drug discovery. There’s nothing new there, they are what scientists need in order to do their job. You are talking about tissue banks, talking about animal models, bio-markers, assays and, interestingly enough, today you also need networking tools – so that the scientists working on CFS can better connect with each other.”
Networking will enable clinicians working in the field to connect with one another and share collected data as well as ideas and not just locally but across the world: ‘It’s an opportunity to cross-pollinate.’
In addition, there should be open access to all the work in the field. Data is not competitive and should therefore be freely made available and shared. Along with the data, our community needs these scientists to join with us as partners, and help work the data. We need established scientists as well as young investigators.
“There are major discoveries waiting to happen in the field and if you offer the tools, if you offer the data, some young scientists, who are by definition smart, will flock to what you’ve created and start to work it. They will find the discoveries that will bring innovation to CFS.”
An MIT study showed that physicians who treat patients for whom no treatments are available have been a powerful source of innovation. These physicians know their patients and the disease and might have hit upon something, some drug treatment that actually works. It is not unheard of, this kind of ‘Darwinian experimentation’ and if this data, these findings and this experience, can be networked, then more informed research can progress to the next level.
Industry’s interest will be stimulated by good data and the community will need pharma, whatever their size, to take the data and the tools that are built and use them in order to come up with new drugs.
“The patients are the people who can help the regulators design better trial protocols as we have seen, for example with HIV, because they know what sort of risk they are willing to take and they are the only ones qualified, in my view, to speak about this.”
He notes that 80% of the time and cost of research projects is spent on generating high quality data, but that if patients provide this data, ‘they will come because it’s a way for them to leap-frog that unrewarding stage and basically multiply their productivity five-fold’. He goes further in saying that passion is a perhaps surprising, but key ingredient in affecting change because it, ‘shortens time-lines, lowers costs and raises the probability of success.’
He considers the example of a child who is dying from a disease and whose parents are so determined, they set about establishing a Foundation, and who become specialists in the disease, and who use their acquired knowledge to attract scientists who often find it hard to ignore them:
“It is hard to say no to parents that are driven by that level of passion, and before you know it, those parents who started with nothing have a research program, they have a scientific advisory board, they have tools and data out there, they’ve got compounds in development and drugs on their way to the market. It happens all the time, it happens with diseases that are much smaller than CFS.”
Money will be needed, he observes, but it’s getting cheaper because of the new models for research such as; open-source, crowd source, virtual pharmacy and others. And he noted, the leadership and passion is also present, in for example, the CFIDS Association who have raised over $30 million since 1987.
“Networks already exist, though some infrastructure is required, but you are not starting from zero like those parents who find they have a problematic child. You are well on your way and it can be done.”
2. Knowledge and Intuition to Reposition Drugs for CFS
 Suzanne Vernon, PhD, Scientific Director, CFIDS Association of America [Timestamp: 31:35-60:40]
Suzanne Vernon, PhD, Scientific Director, CFIDS Association of America [Timestamp: 31:35-60:40]
“I think we’re at a very important time… where we can really take this incredible knowledge that we have accumulated over the years in the basic research arena, and use that to really make remarkable advances in CFS.”
Dr. Vernon began working with CFIDS in 2007 following 10 years at the CDC when her involvement with CFS and identifying the biological underpinnings of the condition really began. The original CDC program is ongoing but to some extent initial efforts were hampered by technology. For such a large patient population it is vital to amass and analyze quality data effectively, and in today’s world technology is much more capable and is helping researchers move the field forward.
“We have, over the past 5 years, been very strategic in positioning ourselves as a translational research leader. Essentially creating a bridge between the basic research, the laboratory, the epidemiology, and the bedside; helping to provide infrastructure for basic researchers to bring their discoveries to impact patient communities.”
This information flow– from bedside to research to treatment – is known as de-risking science and its overriding aim is to attract the pharmaceutical industry and develop drugs and treatments. By building an infrastructure to facilitate this flow of data you are effectively taking the risk – the cost – away from those who will work to develop treatments. You are making it far more attractive to work on CFS because you are providing pharma with vital pieces of the puzzle: the data.
One of the infrastructures created by CFIDS is the SolveCFS BioBank and this features a sample repository and registry. The registry, which has only been in development for 3 years, includes 800 ME/CFS patients and healthy controls. CFIDS has already partnered with pharmaceutical companies who are interested in the data this project will generate, proving that ‘if you build it, they do come’.
CFIDS have also built a knowledge and data analysis sharing platform – an open resource that will be launched in the coming months – as a tool for the community, facilitating the accumulation, analysis and sharing of data. Dr Vernon said that very soon every publication that has ever been printed over the years about CFS will be made publicly available online ‘at the push of a button, indexed and everything’.
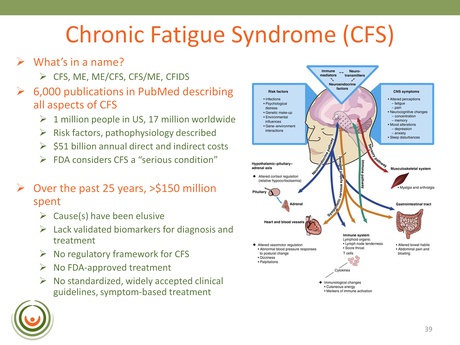
“Over the past 25 years there has been about $150 million dollars spent by the Federal agencies on CFS to basically…gather all that data shown above. Still, a cause, or causes, have been elusive. We still lack validated replicated markers for diagnosis and treatment.”
Dr. Vernon explains that to date there are 6,000 PubMed publications describing aspects of CFS – identifying the risk factors and pathophysiology – and yet physicians are still grappling to manage their CFS patients and can prescribe only symptom-based treatments.
What we do have, she purports, is a foundation of knowledge and experience, upon which we can build, and that drug re-purposing is ideally suited for a complex multi-factorial disease such as CFS that has a clear and unmet medical need. Symptoms are still defining CFS and patients can only at present be treated with drugs that attempt to relieve these symptoms; drug re-purposing will locate and reapply already known drugs to the CFS population.
With this in mind, CFIDS searched for a partner to carry out the work, and found it in a small biotech company called Biovista. Biovista’s platform is the trademarked ‘Clinical Outcome Search Space (COSS)‘ and using this they have been able to access all the data that is on the internet, including all of PubMed and other sites, to create a massive data-set.
Biovista then do a query-search that is specific to CFS with precise sets of search terms in order to create unique profiles, fingerprints or barcodes. The searches can not only be done to locate appropriate drugs but can be used for other applications like finding biomarkers. This data is then manually curated to reduce it to a ‘list of very plausible sets of information and associations.’
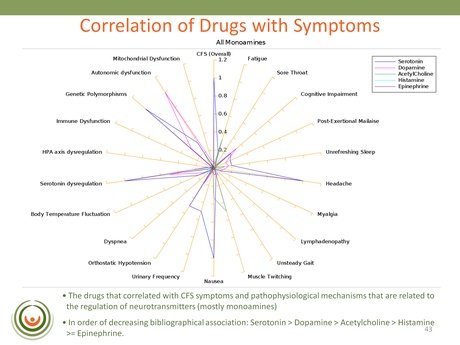
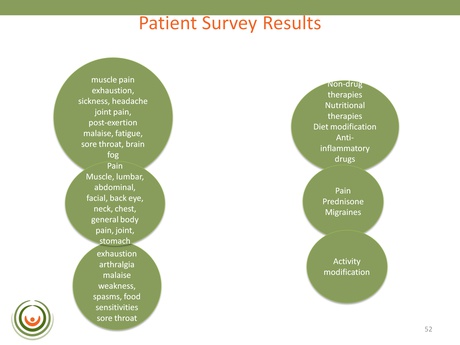
This slide is an example from one of the searches that looked for associations between symptoms, markers and mechanisms in the published literature. One of the strongest correlations identified was with Serotonin, Dopamine, Acetylcholine and Histamine (in that order): revealing a really strong association with monoamine neurotransmitters.
Biovista continued their search by looking at CFS and adverse events – essentially unwanted and reported side-effects. They found that with certain serotoninergic and noradrenergic drugs there was a reported greater frequency of exhaustion and chronic fatigue than with neuroleptics; and this was confirmed using the FDA’s AERS database. The obvious concern for patients here is that these adverse events could be contributing to the symptom severity of the illness; and who wants to take a drug that is known to exacerbate one or more of their main symptoms?
In summary Dr Vernon said of COSS that CFS symptoms and pathophysiology correlated with known mechanisms of neurotransmitters and that these were associated with fatigue. She said that this finding was ‘actually fitting quite nicely with a mechanism that is being extensively described in the literature and known as the ‘Central Fatigue Hypothesis’’ [the link provides an example of this pre-existing theory].
COSS has also identified two drugs – that unfortunately Dr Vernon was not able to name – that could “be used in combination to address two to three CFS symptoms. The knowledge base identification of these drugs was validated, both by the adverse event databases and by data in our SolveCFS BioBank, and we are currently in the process of designing a Proof of Concept clinical trial to test these drugs as a combination therapy. So, a very exciting result from a partnership that literally started one year ago.”
Drug repositioning requires access to and the extracting of data from the clinical population; another untapped resource of knowledge:
“…the key is that a lot of the researchers in this [field] to date have been out there on their own. They’re clinicians who are following a series of patients for decades. And no-one’s been able to tap into the kind of information that they have…” Dr Kweder as quoted by Dr Vernon.
Following a meeting with Dr Unger at Cold Harbor in October 2012, CFIDS along with Biovista developed a web-based tool to capture this ‘clinical intuition’. It listed all the medications that are prescribed according to the literature and all of the symptoms these therapies are designed to target.
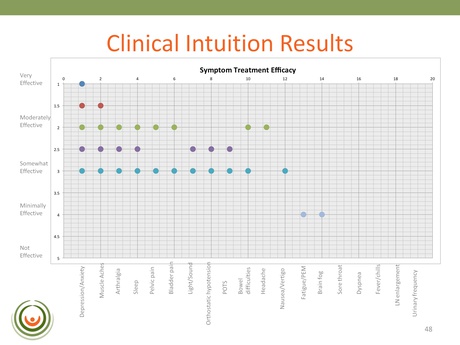
This resource was designed for physicians to complete, enabling them to indicate whether or not the drug actually had the efficacy level hoped for; and then the results could be analyzed. CFIDS sent this out as a questionnaire to 30 physicians in order that they could rate the value of these drugs based on their experience. After a long time and a lot of effort the 50% response rate proved very valuable:
The slide here indicates only those drugs that displayed a somewhat-effective to very-effective level of efficacy. The two drugs identified using the Biovista COSS platform – that are being looked at for the proof of concept clinical trial – are not listed here but were validated by the results.
One of the other things noted by clinicians in the survey was that Vitamin B12 injections appeared to help with reported brain fog although it had a transient effect. Dr Vernon pointed to the possible impact of observed differences in the gut microbiome of CFS patients and healthy people, and how this could impact perhaps on the fermentation and bio-availability of B12 in the body. This suggests that these injections were boosting a depleted system. CFIDS were hosting a webinar about the gut microbiome the following week.
Another connection was reached when both COSS and the clinicians identified two predominant subtypes in the CFS population – the immune phenotype and autonomic phenotype. Clinicians also went on to highlight a further subtype and that was the one existing between older and younger patients.
Finally, Dr Vernon talked about the CFIDS Drug Development Survey. This comprised a 20 question open-ended-text questionnaire that they provided to the FDA to send out to patients. The questions related to disease impact, symptoms and treatment and they have begun to analyze the 1,200 responses – some 500,000 words – with very sophisticated software that has matched the words with unified medical language, and are still in the process of looking more closely at the clusters and the underlying information.
High level results on this slide, revealed three predominant groups of symptom responses (on the left) and treatment responses (on the right): “The message from this is that clearly the patient population is providing information…that can allow us to begin to subtype, [to] identify phenotypes we might not have found otherwise. There was not as much information from the treatment realm.”
Read more: Survey Highlights Serious Unmet Need for Safe and Effective Treatments 29 May 2013
CFIDS will continue with preparation for the Proof of Concept trial and with other trials work, and expand the SolveCFS BioBank initiative which will provide data for these trials. They will continue to optimize the clinical intuition platform and reach out to physicians to try and better understand phenotyping and co-morbidity. And they will seek to use some of the passive and active patient data initiatives mentioned by Dr Munos – such as Fit-Bit – as well as making available the data sharing platform so that collected information can be accessed by everyone.
3. Drug Development and Review: FDA’s Expedited Programs for Serious Conditions
CDR. Melissa Robb, Associate Director for Regulatory Affairs, Office of Medical Policy Initiatives, Office of Medical Policy, Center for Drug Evaluation and Research, FDA [Timestamp: 61:15 – 78:26]
“…‘serious conditions’ are conditions associated with morbidity that have substantial impact on day-to-day functioning. The FDA view ‘serious’ to be broader than ‘life-threatening’, but life-threatening diseases would be considered ‘serious’ also…”
This part of the conference underlined why our disease is receiving the greater focus of the FDA: the recognition that CFS is a ‘serious condition’ and the granting of Expedited Program status for drug development. The unmet clinical need is now formally realized and supported efforts are being made to better facilitate the creation of new drug therapies.
“…we’re looking at other ways to provide the needed evidence so we can be assured that drugs that are approved are both safe and effective and have appropriate risk benefit assessment for the diseases that they are intended to treat.”
Cdr. Robb explained much of the regulatory framework behind this drive of the FDA to support innovation and expedite drug development. She spoke about the 2012 FDA “Safety and Innovation Act,” (FDASIA-title IX) which clarified the requirements for Accelerated Approval and created a new type of program: Breakthrough Therapy.
The Act comprised four programs:
- Fast track designation
- Breakthrough therapy designation
- Accelerated approval
- Priority review
Eligibility for Fast Track status requires data that demonstrates a potential to meet an ‘unmet clinical need’. Traditionally, sponsors would have to submit the complete package of data and analysis to support their application, but with Fast Track they can submit parts of the package as they become available: “the goal is to expedite the review process and get the drug to market quicker.”
Where preliminary clinical evidence already exists – indicating that a proposed drug shows substantial improvement over existing therapies – then Breakthrough can be utilized. This program provides more intensive guidance on drug development, with a cross-disciplinary review that seeks to ensure the whole package is ready to market as soon as possible following approval. Breakthrough is new although it has been used in rare disease drug development and aims to reduce the development time-line.
While Fast Track and Breakthrough are concerned with making the drug development process easier, Accelerated Approval is concerned with the clinical ‘endpoint’ – how a drug’s effectiveness, its success, is measured during the trial. Where it is not possible to measure morbidity or mortality – because you want the drug on the market quickly – intermediate endpoints can be substituted that can predict a clinical benefit.
The endpoints used in Accelerated Approval are therefore different from those used in the more traditional approval process and do not take as long to measure. Accelerated Approval has been used extensively in cancer and HIV drug development.
“…you have to show two things with Accelerated Approval: that the end point you have demonstrated is reasonably likely to predict the benefit; and that there is safety and efficacy with that end point.”
Finally, Cdr. Robb considered the Priority Review Designation that aims to shorten the time FDA has to review an application. Typically applications are reviewed within 10 months – the Standard Review – and for those afforded Priority Review Designation, additional resources are allocated to cut that goal to 6 months: “For CDER, the criteria is that you need to demonstrate a potential to be a significant improvement in either safety or effectiveness…”
“It is important to note that these programs are not mutually exclusive. We do see many drug development programs that take advantage of more than one of these. And that’s great. That’s what they’re intended to do. They all have different contributions they can make to drug development and getting patients access to these important life changing medications sooner.”
4. Audience questions and answers [Timestamp: 80:42 – 96:21 End]
There was not a lot of time for questions to be addressed, although a full transcript of what was said is available on the link at the top of this article. Included below are the first four exchanges abridged where necessary for space and clarity.
Question 1
Dr Vernon to Dr Munos: “I think that you alluded in your presentation to the fact that CFS appears to be on the right track as far as being able to be innovative and expedited in a disruptive way. What kind of time frame would you put on us being a Chordoma or an MMRF or one of those organizations that clearly is innovative and doing good things?”
Dr Munos: “If you go and try to develop a drug the traditional way, you are talking about a 10-15 year timeline, which is probably more than the patients in this room would like to see. If you go the drug re-purposing route the hope is that you can bring innovation to a patient within 5 years. This can be speeded up if patients do join hands and collaborate and work together to help make it happen….”
Question 2
Audience question to Dr Munos: “Do you have any suggestions about the networking part to cut across silos and work collectively?”
Dr Munos: “I think you can have networking at two levels, at the level of the scientists, the physicians, and also at the level of the patients. And to be effective I think those two levels need to cross-pollinate with each other… When you’re having [networked] ideas then [innovation] starts to emerge as if by magic. That’s a dynamic we need to put in motion. I think we have physicians that are clearly involved in CFS, maybe they’re not as networked as they should be – maybe they don’t know each other, they don’t cross-pollinate enough – and we’ve clearly got patients who are activists, who are agitating out there, but we’ve got a million people in this country who are affected by CFS and we need to draw far more into these efforts than seem to be currently involved.”
Question 3
Audience question to Dr Vernon: “You searched only for fatigue and exhaustion in the Adverse Event Database. So it’s not surprising that you came up with neurotransmitters. Have you looked for Orthostatic Intolerance, multiple infections or abnormal immune functions? It seems to me that you are biasing your results by your choice of search terms.”
Dr Vernon: “I’m actually not as familiar with what the Adverse Events database’s information has in there as far as the types of adverse events listed. Can you guys help me out on that?”
RADM Kweder: “There are thousands. Thousands of terms. There’s a whole dictionary of terms. So you can go in and search for others.”
Dr Vernon: “So that’s a very good point, and, yes, there probably is some bias to that search, but the result was pretty clear that fatigue and exhaustion was associated with the mechanism of action of these types of drugs. And I think that’s a really good point to go back into the data and do the same type of searching for adverse events associated with other symptoms. Yes, a very good point.”
Question 4:
Audience question to Dr Vernon: “It is known that the quote CFS end quote literature includes studies based on overly broad and non-specific definitions. As a result, these studies include patients who don’t have the disease that patients described yesterday [during Day 1]. Did you eliminate those studies from the Biovista analysis, and if not how has that influenced your results?”
Dr Vernon: “It used all information. The [database comprised] 20 million abstracts and 250,000 full text articles. So the search was based on terms that are used to describe CFS and a number of biomarkers and the kind of physiologic parameters that have been used to describe CFS. So it’s really a very agnostic approach to trying to find associations: tapping into all kinds of knowledge that describe the possible mechanisms that could help us explain the physiology of CFS.”
RADM Kweder: “So you didn’t try and cull out the articles in the first place, what you did – as I see it – is you cast a very, very wide net. And in that process, you’re going to get – you are going to start to see conditions or symptoms, signs and symptoms coalesce into groupings and that’s ultimately what you found.”
Dr Vernon: “That’s right.”
RADM Kweder: “And you found that actually there’s probably more than one condition or there’s more than one sub-type of the condition which is what we heard yesterday, particularly from some of the clinicians, so this is a heterogeneous group of subsets.”
Dr Vernon: “I think maybe the way the symptoms and the signs that we use to set up the original search terms are inclusive of those that are outlined in the Canadian criteria and the International criteria. So we tried to be as inclusive as possible.”
Still to come in this series of articles:
Day 2: Panel 2: Symptoms and Treatments: A View from Clinicians and Patients: Video 6
Day 2: Panel 3: CFS and ME Clinical Trial Endpoints and Design: Video 7
Day 2: Panel 4: Roundtable Discussion – Summary and Path Forward: Video 8
It is highly likely that given the time which has now elapsed since this conference was held, and the coverage elsewhere, we will only review and possibly edit the transcripts accompanying these videos and look to publish these along with a short overview.
New FDA Transcripts
As we were working to produce this article the FDA went ahead and published new versions of their transcripts that we will try to utilize in our future efforts: