 Could the first drug for ME/CFS be a chemotherapeutic drug? That would sound like a long shot at best but the fact is a two physicians in Norway are having surprising success with a drug originally developed to treat cancer.
Could the first drug for ME/CFS be a chemotherapeutic drug? That would sound like a long shot at best but the fact is a two physicians in Norway are having surprising success with a drug originally developed to treat cancer.
Their Story
Dr. Fluge and Dr. Olav Mella of Haukeland University Hospital in Bergen, Norway, began their investigation of Rituximab’s effects on CFS after treating several Hodgkin’s Lymphoma patients who had long standing cases of CFS prior to developing cancer.
To the doctors’ (and the patients!) surprise the three people with ME/CFS reported they began experiencing significant improvement in their ME/CFS symptoms some 6-12 (16) weeks after completing their chemotherapy regimen. The significant effects lasted up to six to 16 weeks with benefits still present at 6 months. Eventually, however, they relapsed. A second trial resulted in a similar pattern of increased health, this time of a bit longer duration followed again, by a relapse. A third course in two patients evoked a similar pattern.
These successes prompted the physicians to begin a small 30 person Phase II double-blinded placebo controlled trial which began in June 2008 and ended in June 2010. (Phase II trials analyze safety and efficacy)
Very Severely Effected Patients – Reporting that “Based on pilot patient observations, and experience from the prior study KTS-1-2008, the investigators anticipate that severely affected chronic fatigue syndrome patients may benefit from B-cell depletion therapy using Rituximab “ the physicians then began a second phase II trial of very severely effected people with ME/CFS. This longer and considerably smaller) trial began, apparently in an effort to see if the regimen could result in a more permanent state of health. This trial consists of 10 patients and will last until December, 2013 with the drug being given at 3, 6, 10 and 15 months.
Less Severely Effected Patients – A larger study (n=30) containing less severely affected CFS patients, which has not begun recruitment yet, is slated to end in Dec, 2013 as well.
What is Rituximab?
A New Class Of Drug – Developed by IDEC Pharmaceuticals, Rituximab is the first of a new class of drugs called B-cell depleting monoclonal antibodies. It was approved by the U.S. Food and Drug Administration in 1997 for B cell non-Hodgkins lymphoma patients who were resistant to other chemotherapy regimens. Now part of the standard therapeutic protocol for many B cell lymphomas, it is also used in the treatment of leukemia, myelomas and many autoimmune disorders including Rheumatoid arthritis, autoimmune hemolytic anemia, vasculitis (for example Wegener’s Granulomatosis), type 1 diabetes mellitus, Sjogren’s syndrome and several others.
Rituximab is currently co-marketed by Biogen Idec and Genentech in the U.S. and by Roche in Canada (under the trade name Rituxan) and the European Union. Its success in several diseases opened up a new field of drug development with several B-cell depleting monoclonal antibody drugs being produced or currently under development.
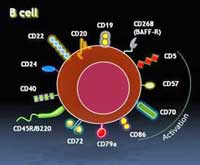
 B-cells
B-cells
Until they are activated B-cells quietly troll the blood, collecting and digesting molecules called antigens that appear to be suspicious. Once they are digested bits of the proteins are placed onto MHC molecules on their cell surface for T-cells to inspect. If the T-cells decide those molecules came from a pathogen, they turn the B-cells on – transforming them into antibody producing machines (‘plasma cells’) that can generate from 100’s to thousands of antibodies per second.
These antibodies or immunoglobulins are specifically manufactured to lock onto a portion of a pathogen or a cell. This accomplishes several things. In order to get into a cell, viruses, fit, like a key into a lock, onto receptors on a cell – a process that antibodies disrupt. Attaching themselves to a pathogen is also a red flag for macrophages to come gobble the pathogen up. Antibodies also turn on the complement system – which can destroy pathogens as well.
B-cells also produce powerful inflammatory cytokines that trigger the immune response and the activation of other immune cells. They are key players in the immune response and overactive B-cell activity has been implicated in auto-immune disorders.
Monoclonal Antibodies – monoclonal antibodies are identical antibodies that target one specific protein on a cell. Their ability to do this has lead to their use in diagnosing pathogens, guiding medications to specific cells in cancer cells, etc.
B-cell Depleting Antibodies – target and attach to B-cells. Rituximab is a monRituximab, B-cells, NK cells and T-cells and Treating CFS (ME/CFS)oclonal antibody that targets the CD 20 receptor and kills B-cells that have this receptor. It depletes the body of B-cells. The CD 20 receptor shows up on the surface of mature B-cells and appears to play some role in turning B-cells into plasma or antibody producing cells.
Like so many other drugs researchers do not know exactly how Rituximab depletes the body of B-cells but they have some ideas. They believe that Rituximab binding to B-cells may signal natural killer cells, macrophages and moncytes or the complement system to kill the B-cells. Rituximab binding could also simply tell the cells themselves to commit suicide. It may be that in different diseases different pathways come into play.
Reason”s the CFS Patients Improved
None of this, of course, overtly suggested Rituximab might be effective in ME/CFS. Yes, Dr. Peterson has identified a non-Hodgkins Lymphoma subset in Incline Village but that paper (a year and a half later!) has yet to be published. There have been smatterings of concern about the auto-immune component in ME/CFS yet it’s never been much of a research focus. Rituximab’s ability to reduce B-cell numbers, however, presents several intriguing possibilities given what is known about CFS.
Viruses
Herpesviruses — One of the more intriguing connections involves herpesviruses. Herpesviruses infect B-cells and replicate in great numbers during B cell transformation. Killing B-cells before they get ‘transformed’ or activated could reduce or even cause a herpesvirus infection to die out as the B-cells they have infected die over time.
Rituximab has been studied in EBV related diseases and two studies underway appear to be focusing on its effects on herpesviruses.
The NIAID awarded Marcia Blackman at the Trudeau Institute $235,000 to examine B-cell depleting antibodies and herpesviruses in mice with an eye towards stopping the development of B-cell lymphomas in transplant patients. The broad research community has yet to embrace the importance of herpesviruses in ME/CFS but there is no doubt as to their virulence in other areas. The immunosuppression organ transplant patients require increases their risk of EBV associated B-cell lymphoma.
 Rituximab, B-cells, NK cells and T-cells and Treating CFS (ME/CFS) – Dr. Blackman’s goal is to determine whether a b-cell depleting antibody can ‘lower the latent load’ or even to ‘purge latency from the host’; ie to completely cleanse the mice’s system of herpesvirus infected cells. Dr. Ann Arvin at Stanford also has a grant to study “Protective Immunity against herpesviruses” that may appears to involve Rituximab as well. https://projectreporter.nih.gov/proje…0&icde=5938112
Rituximab, B-cells, NK cells and T-cells and Treating CFS (ME/CFS) – Dr. Blackman’s goal is to determine whether a b-cell depleting antibody can ‘lower the latent load’ or even to ‘purge latency from the host’; ie to completely cleanse the mice’s system of herpesvirus infected cells. Dr. Ann Arvin at Stanford also has a grant to study “Protective Immunity against herpesviruses” that may appears to involve Rituximab as well. https://projectreporter.nih.gov/proje…0&icde=5938112
XMRV? – the recent announcement from Spain that XMRV has been found in B-cells suggests that, if that finding is validated, Rituximab’s effectiveness in some people could be due to reducing XMRV loads. If XMRV works out this could, in fact, be a more viable explanation than herpesviruses because the positive XMRV study results suggest XMRV may be more prevalent in the ME/CFS population than herpesviruses.
An Auto-immune Disorder?
Rituximab is now being used effectively in a variety of autoimmune disorders and is being studied in even more. Could Rituximab’s success mean that CFS is, in fact, an autoimmune disorder in which some as yet unidentified tissues are being attacked? The purported Th2 shift in the immune response found in CFS which may result in increased antibody production – could be interpreted as being on a step on the road to an autoimmune disorder.
The increased Th2 immune response could also contribute to the NK and T cell ‘burnout’ that’s characterized by low levels of perforin – a substance NK and T cells use to kill infected cells. Because B-cells produce inflammatory cytokines such as TNF-a that turn on the immune response then reducing B-cell populations could conceivably normalize the immune response returning the person to a better state of health.
B-Cell Depletion- Or Something Else?
The case for B-cell cell depletion may have a few holes in it. Studies show that, in general, improvement in rheumatoid arthritis and other autoimmune disorders with Rituximab is associated with a ‘profound’ depletion of all B-cell types, and that relapse occurs in RA patients whose B-cell repopulation is accompanied by higher numbers of ‘memory B-cells’.
Some people with auto-immune disorders have immune responses that don’t seem to fit the B-cell depleting scenario, however. Rituximab appears to be able to positively affect some people with auto-immune disorders without affecting the level of auto-antibodies produced (antibodies directed to attack the body). The improvement in ME/CFS and rheumatoid arthritis patients takes place long after B-cell levels dropped. The fact that many patients relapse long before their B-cells return to normal levels suggests that Rituximab is effecting other parts of the immune system as well.
Rituximab and CFS – B-cell Depletion? If Rituximab is making them better without affecting their B-cells just what is it affecting? The answer may be a hopeful one for people with ME/CFS. Some researchers believe Rituximab may be affecting other immune cells/processes, some of which may be implicated in CFS.
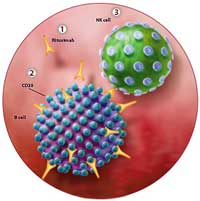 Kessel notes that subsets of both natural killer (NK) and T-cells carrying the CD20 antigen are probably reduced as well by Rituximab. Rituximab also reduces levels of a receptor (CD 40) (in one study 4-fold!) that activates T-cells and promotes inflammatory activity, which suggests that Rituximab may be lowering inflammatory activity in body in several ways. Rituximab also reduces levels of the CD 69 and HLA DR receptors.
Kessel notes that subsets of both natural killer (NK) and T-cells carrying the CD20 antigen are probably reduced as well by Rituximab. Rituximab also reduces levels of a receptor (CD 40) (in one study 4-fold!) that activates T-cells and promotes inflammatory activity, which suggests that Rituximab may be lowering inflammatory activity in body in several ways. Rituximab also reduces levels of the CD 69 and HLA DR receptors.
Macrophages were more effective at killing leukemia cells following Rituximab treatment in one study. Rituximab also appears to increase macrophage production of IL-10, an important anti-inflammatory cytokine that is sometime increased in CFS. The production of tumor necrosis factor alpha (TNF-a), one of the most potent inflammatory cytokines, and one which some researchers think plays a role in CFS, was also reduced in Rituximab treated macrophages. Interestingly, it appears that as B-cell levels reduced, macrophages with anti-inflammatory characteristics may increase – thus providing better immune protection with less inflammation. The overall effect may simply be of reducing inflammation in a number of ways.
Natural Killer Cells – a more pertinent finding for ME/CFS might be a significant improvement in NK functioning. Interestingly, a small number of natural killer and T-cells also express the CD20 receptor that Rituximab binds to, and Rituximab therapy, not surprisingly, wipes out this subtype of T and NK cells. One study, however, found that monoclonal antibodies activated NK cells earlier – possibly improving the immune response. Another found that using Rituximab in combination with another monoclonal antibody significantly enhanced NK cell degranulation and cytotoxicity – ie killing ability -a key problem in CFS.
The CD 69 marker is intriguing because it signifies that the NK and T cells have been activated by a pathogen. Interestingly, levels of the CD 69 receptor may be reduced, not increased, in CFS. In fact, Maes et al suggested that low CD 69 levels may contribute to increased pathogen prevalence in CFS.
Dr. Olav Interview
As you point at, our time is limited. We will briefly give some answers, as we have kept a low profile after our publication in BMC Neurology last year, but have had many mails from patients around the world, some of which we have had the opportunity to answer, others not. Some patients are trying to attain information and others are volunteering to participate in our trials. The CFS project is a side study in a very busy university oncology clinic and resources thus limit rapid progress. However, we find working with these previously often neglected patients rewarding and believe there may be links also into our mainstream oncology field of work.
How effective has Rituximab been? Would you say the general response has been improved quality of life but still with quite limited functionality, improved with significant increases in functionality, improved approaching wellness?
The Rituximab effects in CFS patients ranges from no effect or some effects below the threshold for being categorized as a response in a clinical trial, to major responses with a total change in quality of life up to feeling near normalized concerning symptoms and also function in daily life and work. As stated in the pilot study in BMC Neurology in 2009, the responses after two Rituximab infusions (2 weeks apart) in most patients last for some time, the symptoms gradually coming back. The results of our double-blinded, randomized study is not yet published and the results will have to await the paper. Therefore the response rates can presently not be stated.
Do you have any clarity as to why Rituximab had positive effects on some people with CFS? Do you believe that it’s more likely to due to the reduction of EBV infected cells or antibody/autoimmune effects or immune modulation of T and/or natural killer cells?
Rituximab and EBV infection in CFS – We can only partly answer so as not to disclose unpublished data. We have no proof of the mechanism of action of the B-lymphocyte-depleting agent rituximab in CFS. However, the mode of action of the drug makes us believe that modulation of the immune response in patients is a major part of the mechanism. The quantitative effect on the different elements in the immune system and which seems to be important in CFS patients, is being investigated.
Finally, virus as possible etiology has recently achieved much attention in CFS. There does not have to be a discrepancy between that and what we observe. Autoimmune processes often may have precipitating infections with virus or bacteria. Our belief is that in CFS elucidating and targeting the immunological response in the patients is an important clue to better treatment.
(The idea that autoimmune processes can be precipitated by a viral trigger is a solid one. Some researchers think a viral attack on the myelin sheathing the nerves confuses the immune systems of some people into ultimately attacking the neurons themselves – and that this is what causes multiple sclerosis. Interestingly, two herpesviruses, EBV and HHV6, are prominent viral candidates. MS is also a relapsing-remitting type disorder (although much more so than CFS) highlighted by severe fatigue. )
Do you believe that repeated courses of Rituximab could lead to a more permanent state of health? Is that why you are doing the repeated courses trial?
The reason for the ongoing trial with repeated rituximab infusions is of course the observation that responses tend to be transient, possibly related to the inevitable rise in B-lymphocytes. When we now are doing an open Phase II study with repeated rituximab, this is based on presenting the Ethical Committee with some pilot data using this concept.
Are you gathering data on any of biological markers that might explain Rituximab’s effects on CFS such as B-cell levels, the status of EBV infections, NK cell functioning, CD20, CD40, CD69, HLA DR levels in NK/T-cells or levels of TNF-a or IL-10?
Yes, biological spin-off studies are a major part of our effort and we are analyzing how the biological parameters in the intervention group with rituximab are changing compared with the placebo group. The need to harvest biological material sequentially during the study is a reason for not being able to include patients from other parts of our country or the world at large.
Do you know of anyone else experimenting with Rituximab in CFS?
We do not know of anyone else working systematically with this class of drugs (like rituximab) in CFS. However, we have had patients sending us personal reports of being better from CFS when they were treated for other conditions (as for example indolent lymphoma) with rituximab.
Are there other drugs in this class that might be as or more effective?
 We strongly believe that B-lymphocyte actions in some way are a part of the explanation of the symptoms in CFS (at least in a subset of patients). Therefore one would expect that other drugs influencing B-lymphocyte numbers and function might improve CFS symptoms. However, there are no trials to our knowledge looking at this. We will point to our pilot study in BMC Neurology last year (Fluge and Mella, online free access), were we explained that methotrexate in a cytotoxic chemotherapy combination was the clue to our reasoning that B-lymphocyte depletion might benefit CFS patients.
We strongly believe that B-lymphocyte actions in some way are a part of the explanation of the symptoms in CFS (at least in a subset of patients). Therefore one would expect that other drugs influencing B-lymphocyte numbers and function might improve CFS symptoms. However, there are no trials to our knowledge looking at this. We will point to our pilot study in BMC Neurology last year (Fluge and Mella, online free access), were we explained that methotrexate in a cytotoxic chemotherapy combination was the clue to our reasoning that B-lymphocyte depletion might benefit CFS patients.
You mentioned Rituximab is something of a side project. If you had more funds could you move more quickly? What would you need to do so?
I believe recruiting patients to our present two studies is going well and is as fast as our time and resources permit. The part which may limit us is analyzing and working out all the biological data we accumulate from the patients sequentially during the time course before and after the treatment (up to three years in the present studies).
We have the know-how to do advanced analyses that may give us clues to the actual mode of action of the B-lymphocyte depleting agent in CFS-patients. Our working hypothesis is that the main functional disturbances in the patients may be explained by an autoimmune mechanism. The data we have seen during the year passed since our pilot study publication we believe is well in accordance with that.
(In 2009 the CFIDS Association of America applied for two grants to study auto-immune processes in CFS. Demonstrating substantial interest in this area one of the grants included researchers from Walter Reed Army Medical Center, Oklahoma Medical Research Foundation, Harvard Univ. , New York University, Univ. Of Wash, Univ. of Chicago and the Univ of Miami. (!). (This may have been the most widely supported grant proposal in ME/CFS history ) Both grants, alas, were turned down – but what an interesting light they might have shown on these Rituximab findings).
The support we would need most is any donation that might support hiring a molecular biologist to help speed up these ongoing studies. It is quite extensive work trying to map out what is happening with genes, cytokines, immune cells etc.
Serious Diseases – Serious Drug
Perhaps not surprisingly for drug developed as a chemotherapeutic agent Rituximab can have significant side effects.These are rare but can include severe breathing problems, heart problems, serious even potentially fatal skin reactions (Steven’s-Johnson syndrome), a brain infection (Progressive Multifocal Leukoencephalopathy) and kidney problems. Rituximab is not cheap as well, with a two dose course for rheumatoid arthritis clocking in at about $11,000 a year.
The Answer For CFS?
Rituximab with it’s high costs and, at least thus far, temporary effects, may or may not be the answer for ME/CFS but, at the very least, if the findings are validated it will point a arrow straight at the immune system thus bolstering more research and interest in it.
The incredible interest in B-cell depleting antibody drugs such as Rituximab could also bode well for ME/CFS. The government clinical trials database lists, believe it or not, 977 studies on Rituximab on disorders numerous types of cancer, auto-immune disorders (rheumatoid arthritis, lupus, Sjogren’s Syndrome, ankylosing spondylosis) and many rare disorders (Pulmonary Alveolar Proteinosis, Waldenstrom’s Macroglobulinemia, etc.). (I actually paged through several hundred of them thinking there can’t be this many studies on one drug – but each one featured Rituximab)
Rituximab’s success has spurred the development of more and purportedly more powerful B-cell depleting monoclonal antibody drugs such as Orelizumab, tositumomab, Ofatumumab and Belimumab with others (RTX, MDX-1100) in development. This is a wide open and vigorous field – a very good one for ME/CFS to latch onto, if it can.

 B-cells
B-cells