Discuss this article on the forums
 Stating that there’s been a ‘major revolution in science… brought about by technological advances that have allowed us to look at data generated in the lab in a different way” Dr. Basil Aldadah kicked off the Systems Biology session. These studies tend to use sophisticated analytical techniques to look at very large amounts of data. They are expensive, resource intensive studies that some people think provide the best opportunity to understand complex disorders like chronic fatigue syndrome. Others wonder, ultimately, how accurate they are.
Stating that there’s been a ‘major revolution in science… brought about by technological advances that have allowed us to look at data generated in the lab in a different way” Dr. Basil Aldadah kicked off the Systems Biology session. These studies tend to use sophisticated analytical techniques to look at very large amounts of data. They are expensive, resource intensive studies that some people think provide the best opportunity to understand complex disorders like chronic fatigue syndrome. Others wonder, ultimately, how accurate they are.
Gordon Broderick (Minute 204) – Regulatory Imbalance in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: A Network Approach There are ups and downs in every conference and Broderick’s presentation, which generated quite a bit of discussion throughout the conference, was definitely one of the ‘ups’. Broderick has been busy over the past year publishing five studies on ME/CFS/GWS (as well as others) and more are on the way. His model of ME/CFS focuses on how the main regulatory systems in the body – the immune system, endocrine system and the nervous system – function and interact (or don’t interact) with each other. Essentially he trying to “map out the wiring” of what he believes is a steady state of “pathological dysregulation” in ME/CFS.
Systems Out of Whack: the HPA Axis and the Immune System – Sophisticated data mining techniques enable Broderick to use ‘biomarkers’ to draw maps that delineate “centers of power” and “networks of connectivity” which display which systems in the body are activated. For instance, using a CDC data set his analysis found unusually high levels of engagement by the pituitary and thyroid but decreased connectivity with the adrenal cortex. This suggested that what is usually a very highly integrated system, the HPA axis, is not as integrated as it should be in PWCs. Since the HPA axis is one of the two main branches of the stress response and is an important immune regulator, this is not a system we want to have out of whack.
(This finding may suggest that the hypothalamus and pituitary are failing to activate the adrenal glands in CFS. Alternatively, they could be failing to respond to signals the adrenal glands are putting out, which is probably where Dr. Holtorf would land, since he believes that the HPA axis dysfunction in ME/CFS mainly takes place place in the hypothalamus and pituitary).
The network map Broderick drew revealed a highly concentrated set of nodes indicating what he called ‘a massive buildup of connectivity’ in the immune system that was characterized by Th2 activation and Th1 suppression – something that suggested a bit of trickery on the side of a pathogen may be going on.
He said, “if I were a virus that would be a neat strategy, head-faking you into thinking I’m an allergen.”
The analysis also picked up signs of impaired natural killer cell functioning. This was all, interestingly, enough, found using the much maligned Wichita (‘CFS-lite’) data set.
Charting Clinical Courses of Disease – Broderick then moved onto a stronger cohort and more recent work – adolescents who came down with ME/CFS after EBV caused infectious mononucleosis. (This is an NIH funded study, lead by Dr. Renee Taylor, using the Fukuda definition) Two years after onset, 95% of the adolescents who came down with infectious mononucleosis (glandular fever) had recovered but 5% had not – and they, of course, were the chronic fatigue syndrome group.
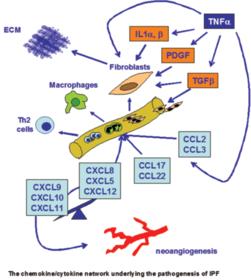 Cytokines, cytokines, cytokines – Because of their ability to cause so many of the symptoms in ME/CFS, cytokines have been a big focus of research. A similar set of studies, called the Dubbo studies, found minor differences in cytokine levels except for very early in the illness. Broderick wasn’t just looking at cytokine levels, however, – he was looking at how the cytokine ‘system’ changed over time and that made all the difference.
Cytokines, cytokines, cytokines – Because of their ability to cause so many of the symptoms in ME/CFS, cytokines have been a big focus of research. A similar set of studies, called the Dubbo studies, found minor differences in cytokine levels except for very early in the illness. Broderick wasn’t just looking at cytokine levels, however, – he was looking at how the cytokine ‘system’ changed over time and that made all the difference.
Three Different Kinds of ME/CFS Jump Out – He was able to use only five cytokines to reveal three distinct illness pathways. One group of patients got worse at first but then plateaued and was very slowly getting better. Another group seemed to be getting better about a year in – and looked to be in pretty good shape, but then just fell off the wagon and basically collapsed; at the end of two years they were the only group that was continuing to get worse. Another group started off poorly but just kept slowly improving; at the end of two years they were not well but it looked like they might get there is the not too distant future.
This is the first study that’s been able to identify different ways this disease progresses. It was impressive he was able to do this using a part of the immune system – cytokines – that researchers have long thought were probably involved in ME/CFS but have had trouble validating. If Broderick’s analysis works out and he can identify key cytokines that influence disease progression and pick out subsets as well – then we will have made an immense step forward. The potential is there for targeting specific cytokines or cytokine groups for treatment.
Three Groups – One General Illness? Although he found three different groups, some evidence suggested they may have been subsets of one general group. Gordon found two cytokines which were present in all the individuals but which, oddly enough, he could not remove from the equation without the pattern collapsing. This suggested (I would guess) that these are key cytokines that, depending on which group you are in, interact differently with other parts of the immune system.
Essentially Broderick seems to be uncovering holes in the immune system that ordinary immune studies wouldn’t have a chance of uncovering. These holes could be allowing pathogens to flourish or they could be activating the immune system to produce ongoing flu-like symptoms… We will hear from Dr. Klimas that identifying immune dysregulation in some patients is allowing her to patch some of those holes – enabling her patients to feel better.
A Deeper Cut – This is cutting edge stuff in itself, but now, Broderick asked, what was causing these dysregulations? Now he shifted gears entirely and dug into the genes these adolescents were expressing to see which gene pathways were being activated (or not).
About 25,000 or so of our genes produce proteins which do most of the work in our body. At any moment the gene expression in our bodies reflects the activities our bodies are engaged in. Exercising will cause gene pathways that support exercise to whip into action, while fighting off a pathogen will cause genes involved in immune pathways to ramp up.
This is where the rubber met the road. Were Broderick’s results a figment of his complex mathematical computations or did the gene expression results match his network analyses? Thankfully, the gene expression results confirmed immune activation (i.e. increased immune connectivity) involving pathways of immune cell signaling as well as pathways associated with natural killer cell functioning.
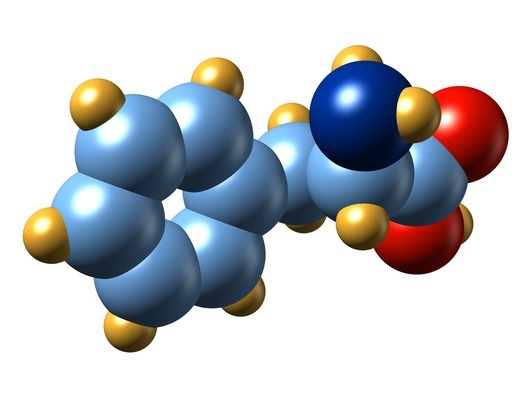 A Metabolic Pathway Gone Awry in ME/CFS? Five pathways were particularly significant and he picked out phenylalanine metabolism as a pathway that may have awry in one group of CFS patients. Phenylalanine is a pretty nice fit because it’s a precursor for tyrosine, dopamine and the two big autonomic nervous system factors, norepinephrine and epinephrine.
A Metabolic Pathway Gone Awry in ME/CFS? Five pathways were particularly significant and he picked out phenylalanine metabolism as a pathway that may have awry in one group of CFS patients. Phenylalanine is a pretty nice fit because it’s a precursor for tyrosine, dopamine and the two big autonomic nervous system factors, norepinephrine and epinephrine.
(What does phenylalanine have to do with the immune system? A 2008 paper suggested that chronic immune stimulation is correlated with reduced phenylalanine turnover. https://pubmed.ncbi.nlm.nih.gov/18781914)
Exercise induced network changes – Broderick then went on to show that in another study exercise induced the neuroendocrine network to greatly increase in size and shape in people with GWS. This suggested that, for some reason, this system was more highly ‘tweaked’ in people with GWS when they tried to exercise than it was in healthy people. If Broderick can dig deeper he should be able to target the key areas of the body that get disturbed when people with GWS or ME/CFS exercise and, indeed, he was able to show that the IL-1 cytokine seemed to be a key player.
Intriguingly and quite promisingly, when he looked at gene expression patterns in these male GWS patients he found that alanine metabolism (remember phenylalanine?) appeared to play a significant role. Broderick said that was ‘quite interesting’ but I imagine he clicked his heels (probably internally) when two separate analyses came up with such similar results… a nice sign that he’s on the right track.
The analysis also highlighted potential problems in detoxification pathways, a subject that gets plenty of interest in the alternative medical community, but not even a hint of interest in the mainstream research community.
Looking at the final pathway visuals, however, it was remarkable how different ME/CFS was from both GWS and the healthy controls with its ‘nodes’ (the central players) jammed together in a small bunch and then flaring out like a horn a plenty into a diffuse network. The picture, which looked a little scary in its strangeness, nevertheless suggested Broderick had indeed gotten hold of a few key players in the disorders – which, who knows, might in the end be the easiest kind of network to fix.
Questions… Broderick’s work is exciting, it gives great visuals, and it’s definitely cutting edge, but does it reflect what is actually going on in the body? It’s easy to see how researchers who expect to see elevated, say, TNF-a levels – and do usually see them when the immune system erupts, are a bit leery about mathematical calculations which show altered ‘association patterns’ but not necessarily high levels of any one cytokine, and there was some grumbling about whether Broderick’s analysis would play out on the ground.
Broderick’s ability, though, to validate some basic immune findings and pluck out significantly different cytokine patterns in people with ME/CFS – where no one had been able to do so before – suggested he was onto something. PLUS, he was able to link those cytokine patterns to different patterns of recovery; i.e. he was able to show that one group with cytokine pattern had this kind of ME/CFS; another group with another cytokine pattern has this other kind of CFS…Plus the two study results tended to validate each other.
Broderick is definitely convincing some people in the NIH that his work is for real because he just snagged one of the biggest grants in ME/CFS history….
 How to REALLY Make a Difference or…how to Turn $5,000 into $4,000,000. At the end Broderick thanked the generous support of the patients and in particular the CFIDS Association, for providing the pilot funding. Later when talking to him I learned how this came about. PANDORA, little PANDORA, with it’s $25,000 or so budget (in a good year), placed a large investment (for them) – $5,000 – on Broderick a couple of years ago. That got him started. He was able to work up some data and approached the CFIDS Association and then got a $100,000 grant.
How to REALLY Make a Difference or…how to Turn $5,000 into $4,000,000. At the end Broderick thanked the generous support of the patients and in particular the CFIDS Association, for providing the pilot funding. Later when talking to him I learned how this came about. PANDORA, little PANDORA, with it’s $25,000 or so budget (in a good year), placed a large investment (for them) – $5,000 – on Broderick a couple of years ago. That got him started. He was able to work up some data and approached the CFIDS Association and then got a $100,000 grant.
Back to the lab he went and worked up more data, and then went for the gold – a ROI NIH grant – and snagged it. This wasn’t just any ROI grant, though, he was awarded a $4 million grant that may very well may be, when all is said and done, the largest single ME/CFS research grant ever. It involves taking a comprehensive look (and we know Broderick’s good at ‘comprehensive’) at everything from cytokines to gene expression to hormones in people with ME/CFS after an exercise challenge.
Patient Funded Research – This is the power of patient-funded research. Patients don’t have the money to fund entire studies but they can fund data gathering efforts that lay the groundwork for major grants. This is what the CFIDS Association and other ME/CFS organizations do. The CAA has an excellent track with at least four of their funded researchers, (Shungu, Huber, Glaser and now Broderick) currently having NIH grants – which is something, given the 8% or so acceptance rate for new CFS grants at the NIH. The Broderick grant alone is several times larger than the CAA’s Researcher Initiative layout – demonstrating an excellent bang for the buck – from that program.
Mangalathu Rajeevan (Raj): Genomic studies of CFS
 Mangalathu (call me Raj) stated that the CDC has been heavily involved in genomics and gene expression work for the past 10 years. Gene expression is still a very young field and is evolving and he pointed out that major breakthroughs had been made in the past year or so.
Mangalathu (call me Raj) stated that the CDC has been heavily involved in genomics and gene expression work for the past 10 years. Gene expression is still a very young field and is evolving and he pointed out that major breakthroughs had been made in the past year or so.
There is obviously no love lost between the CDC and the ME/CFS community, and with the increasing focus on psychology over the past few years that’s only gotten worse; but there is a side of the CFS research team that is heavily focused on pathophysiology and that was what Raj talked about.
Not the Breakthrough We Hoped For…Yet – By digging deep into the gene activity in the body, gene expression was going to, it was hoped, illuminate areas of dysfunction that set ME/CFS apart from other diseases and legitimize the disorder. That has not happened. The gene expression results to date have been decidedly mixed. The 26 papers produced indicate a high amount of interest in the subject but 11 were from one data set (Wichita) and, with the exception of the two Kerr studies, there has been little agreement between the different datasets.
CFS – Bad Gene….Healthy Controls – Good Gene – Raj was able to show that both healthy controls and people with CFS have a certain (bad) variant of a gene called HTR2A but only in people with CFS did that bad gene end up doing bad things. In the healthy controls the bad gene was ignored and the system just purred along. What could cause that ‘bad’ gene to be expressed in people with ME/CFS? Raj postulated that differences in transcription factors, methylation or the stress response could somehow flip the response from being benign (no gene expression) to a potentially disease forming process.
A Look at HTR2A –HTR2A is not a bad gene to consider for ME/CFS involvement. Upregulated neuronal excitation has been postulated, the smooth muscles HTR2A affects line the blood vessels; blood vessel abnormalities are being studied and cytokines may be involved as well.
• CNS: neuronal excitation, behavioural effects, learning, anxiety
• smooth muscle contraction
• vasoconstriction/vasodilation
• platelet aggregation
• Activation of the 5-HT2A receptor produces anti-inflammatory effects
Has something ‘flipped the switch’ on the HTR2A gene causing or contributing to the central nervous system, blood vessel or immune problems in the CDC’s ‘CFS’ patients? Only more research – if it can proceed in the financially strapped CFS research program at the CDC – will tell.
From the HPA axis to Inflammation: a New Focus – Then he turned to some recent unpublished work. The CDC has turned away from their emphasis on the HPA axis to take a closer look at inflammation and immune genes (1,000 genes). They were able to identify 34 polymorphisms (variants of genes) that could contribute to the immune and inflammatory problems in ME/CFS.
Once again (the third time that day; twice in Broderick’s work and once here), the complement cascade was highlighted – an encouraging sign and a clear suggestion to investigate that section of the immune system in more depth. Chemokines (signaling), cytokines and toll-like receptors (pathogen detection) also appeared to play a role. Interestingly, a IL-18 cytokine appeared to play a major role in fatigue. The CDC has rarely done exercise studies but his last slide indicated that he believes they’ll play a key role in elucidating ME/CFS.
This is long term research but the CDC work did validate earlier immune findings and suggested that immune dysregulation, particularly involving the complement, plays a major role in ME/CFS.
Throughout the conference we will hear “inflammation” again and again and it was the topic of the next presentation…..
Dr. Keith Kelley: From Systemic Infection to Brain Inflammation
Dr. Kelley is not a CFS researcher but he was clearly brought in to stimulate thinking about the intersection between something called “sickness behavior” and CFS. The idea of “sickness behavior” was developed out of recognition that it’s the immune system for the most part, not the pathogen, that causes most of the flu-like symptoms we experience when we have a cold. The ‘behavior’ is the aching muscles, fatigue, etc. – none of which do anything to fight off the pathogen – but which sure as heck make sure we stay in bed as much as possible and get our rest.
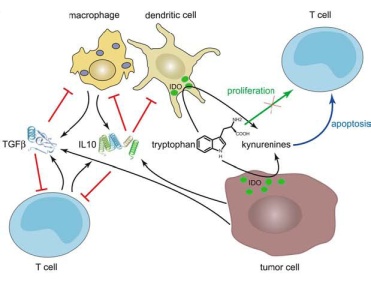 This is all about getting at the biological roots of depression or fatigue or even pain. The key message of Kelley’s presentation was that he has uncovered a factor in the brain (Indoleamine 2,3 Diooxygenase – (IDO)) that appears to determine whether several of the symptoms of ‘sickness behavior’ are temporary in nature (as they are supposed to be) or become a permanent part of someone’s life. Because human brain experimentation is ethically limited, most of this work is done by infecting laboratory animals with a pathogen and then exploring what happened in their central nervous systems. This research nothing to do with the pathogen per se and everything to do with finding a ‘switch’ that gets turned and, instead of resetting itself when the pathogen has been cleared, remains turned on, causing sleep problems, fatigue, depression, etc.
This is all about getting at the biological roots of depression or fatigue or even pain. The key message of Kelley’s presentation was that he has uncovered a factor in the brain (Indoleamine 2,3 Diooxygenase – (IDO)) that appears to determine whether several of the symptoms of ‘sickness behavior’ are temporary in nature (as they are supposed to be) or become a permanent part of someone’s life. Because human brain experimentation is ethically limited, most of this work is done by infecting laboratory animals with a pathogen and then exploring what happened in their central nervous systems. This research nothing to do with the pathogen per se and everything to do with finding a ‘switch’ that gets turned and, instead of resetting itself when the pathogen has been cleared, remains turned on, causing sleep problems, fatigue, depression, etc.
IDO seems to be one of the switches that does exactly this…turn it on and you get a lethargic, sick looking mouse. If I understand it correctly, Dr. Kelley has been able to engineer an IDO deficient mouse which showed no signs of illness after it was infected; it didn’t stop running at night, it didn’t seem lethargic…..that mouse just hummed away because the switch that turned on the ‘sickness behavior’ in the brain – had been removed.
There are undoubtedly several such ‘switches’ in the brain. One, which magnifies the pain response during an injury (good thing) but then ramps it up further (bad thing) even after the injury has been mostly healed, probably ’causes’ fibromyalgia and irritable syndrome and other mysterious pain producing disorders. Somewhere in the CNS probably lies an indoleamine counterpart for pain.
I asked Dr. Kelley if high levels of inflammation were needed to keep this system going or if the central nervous system was so sensitized that it might be responding to normal levels of cytokines or other factors and he said the second was certainly possible. A big question in ME/CFS has been where the ‘trauma’ is….is it in the periphery in the form of high cytokines and low cortisol, etc. or is it in a central nervous system that is somehow misreading the signals from the body? Kelleys work suggests that many of the problems lie in the central nervous system and indeed, ME/CFS, FM and some other disorders are sometimes referred as disorders of ‘central sensitization’.
One wonders if there’s a connection to the Light’s work in all this. They show greatly increased sensory, immune and adrenergic receptor activation in people with ME/CFS. The big question is why are all these receptors – which are presumably sending messages to the brain – on such high alert in people with ME/CFS? As yet there doesn’t seem to be any reason, for instance, for the lactic acid receptors to be upregulated in ME/CFS, because there’s very little evidence of increased lactic acid. But there they are, on high alert and presumably over-responding to the smallest amounts of lactic produced by the muscles. Did a switch in the central nervous system get tripped which activated the alert systems of the body?
Since Dr. Kelley believes these problems are largely immune-based, it wasn’t surprising to see him mention immune agents that he felt might be helpful in fighting inflammation-induced fatigue or pain or depression or other symptoms associated with infections. Dr. Klimas noted that she was trying tumor necrosis factor inhibitors, and there they were right at the top of his list. The list included (262:48)
• TNF inhibitors
• IL-1 receptor antagonists
• Cox-1 and 2 inhibitors
• p38 and JNK inhibitors
• MCP-1 Blockade
• Indoleamine 2,3 Diooxygenase Blockers
• IL-17 and IL-23 Inhibitors
• M2 alternative macrophage differentiation
• Antibiotics (Minocycline, doxycycline, polymyxin B)
• omega three fatty acids
• nutraceuticals (resveratrol, luteolin, flavonoids, cucurmin)
• moderate exercise
• behavioral interventions to increase parasympathetic tone
At least six of these are used by some clinicians in some ME/CFS patients (TNF inhibitors, antibiotics, omega three fatty acids, nutraceuticals, moderate exercise, behavioral interventions.
 Conclusions: the ‘top down’ approach of Systems Biology seeks to uncover problems in system functioning – a far different approach than determining whether cortisol is too low or the TNF-a cytokine levels are too high. This approach presupposes that there are more or less optimal states of system functioning and that by comparing the functioning of systems in healthy controls vs people with CFS – they can identify parts of systems whose wiring has, for whatever reason (genetics, infection, trauma become altered in ME/CFS.
Conclusions: the ‘top down’ approach of Systems Biology seeks to uncover problems in system functioning – a far different approach than determining whether cortisol is too low or the TNF-a cytokine levels are too high. This approach presupposes that there are more or less optimal states of system functioning and that by comparing the functioning of systems in healthy controls vs people with CFS – they can identify parts of systems whose wiring has, for whatever reason (genetics, infection, trauma become altered in ME/CFS.
Although some question whether these complex mathematical analyses really reflect what’s going in the body, a systems approach, if done correctly, should theoretically provide a more accurate look at health (or the lack of it) because health, is, after all, is the product of the functioning of complex systems.
If this complex technology works it should be able to characterize the dysfunctions present in ME/CFS. One encouraging outcome of the systems biology studies to date is that they have tended to validate findings such as the abnormalities found in the HPA axis problems, Th2/Th1 ratios, the complement cascade and natural killer cell functioning.
More from the NIH State of the Knowledge Workshop

