 With no less than four XMLV publications under her belt during the last 14 months Kozak is the premier XMRV evolutionary biologist. Her latest paper is a review of her and others work on murine leukemia retrovirus (MLV) evolution that delineates where contamination could come from and where it could not come from.
With no less than four XMLV publications under her belt during the last 14 months Kozak is the premier XMRV evolutionary biologist. Her latest paper is a review of her and others work on murine leukemia retrovirus (MLV) evolution that delineates where contamination could come from and where it could not come from.
A Hot Item! – The first mouse leukemia retrovirus was isolated in 1951, the next in 1970, from none other than Dr. Jay Levy, a one time CFS researcher, who, believe it or not, scored two papers in Science and one in Nature that year on murine viruses and their connection to cancer – before they were eclipsed by HIV, the mouse retroviruses were hot, hot, hot items.
(Dr. Levy is, reportedly, looking for XMRV in a small number of people with ME/CFS.
Eventually three types of MLVs were discovered:
* MLV’s – infect mice and cause lymphomas and leukemias in them
* Xenotropic (foreign) MLV’ss that supposedly infect other species but not mice. (As the march of science went on we learned this was NOT true, XMLVs (but pparently not XMRV) can infect some mice species. )
* Polytropic MLV’s (PMLVs) – can infect both mice and other species – which would seem to make them the most prevalent mouse retroviruses, but this isn’t true either….XMLVs are actually the most prevalent mouse virus.
A Genome Infected with A Partial Virus – the Endogenous Retroviruses – Forget for a moment about these retroviruses and turn your attention to a degraded form of them which will end up playing a major role in MLV evolution – endogenous retroviruses (ERVs). Retroviruses have a way, over time, of integrating themselves in the DNA of organisms and the MLVs have been no exception. Endogenous retroviruses are too usually degraded and broken down to produce viral proteins anymore….and usually sit in our DNA quiescent, doing nothing….. but that’s not the case with the mouse ERVs; some of them are sufficiently intact to sometimes produce ‘activated’ gene sequences and even infectious viruses.
This puts some mice in a very strange position of not being able to be infected by MLVs but still able to produce them…..That kind of weird logic is all too common in this fascinating family of viruses.
These active mouse endogenous retroviruses are problematic because the presence of mice in research labs means mouse DNA can be present even if the mice are not ‘infected’ with the virus. Any work done with mouse materials that inadvertently ‘wakes up’ an endogenous retrovirus can cause problems. Dr. Kozack noted that “many cell lines in common use carry active ERVs.”
Culturing can be suspect because culturing cells that are derived from mice can cause ERVs to become active and produce either viral proteins or even the infectious virus. Some cell lines produce gene sequences that appear to the researcher to be envelope proteins of an actual virus.
The Endogenous Xenotropic Retroviruses (XMVs)
Of course we’re concerned with the mouse X – ERVs – the endogenous retroviruses most closely related to XMRV – because researchers worried about contamination will most likely turn to these ERVs . Work has recently been done to determine where and how active these ERVs are.
Most XERVs, fortunately, are not able to produce infectious retroviruses but some are. Kozak found two types of lab mice which contain X ERVs that are able to produce high levels of XMLVs (but not necessarily XMRV.) She also found that subjecting some laboratory mice cells to various stressors (chemical induction, bacterial lipopolysaccharides (LPS)) could induce them to produce the XMLV viruses).
She’s been able over the past months to identify four active ‘proviruses’ embedded in mouse genomes that are able to produce XERVs ; one of which (Bxv1) is found in about 1/3rd of the common strains of inbred mice (lab mice?). Bxv1 is nothing to laugh at as it has been associated with leukemias in mice – indicating that endogenous retroviruses can be as dangerous as active, whole retroviruses.
XMLV’s in Mice and Men – She’s identified two strains of lab mice that can produce high levels of XERVs, nine that can produce intermediate levels and four that rarely produce them (Table 2). The two ‘high’ producing strains of lab mice begin producing XMLVs at an early age and it can be easily isolated from their cells and tissues. Other strains of lab mice only begin producing XMLVs after they’ve been stressed with a chemical or immunological stressor.
 At the XMRV Int Workshop in September Dr. Coffin authored an abstract stating that some endogenous retroviruses produced genetic sequences that could be mistaken for XMRV – a cautionary finding for researchers who have not yet produced and sequenced, as the WPI did, the entire virus. Recently Dr. Coffin, however, announced the creation of a test that could distinguish between ERV produced XMRV-like genetic sequences and XMRV produced sequences.
At the XMRV Int Workshop in September Dr. Coffin authored an abstract stating that some endogenous retroviruses produced genetic sequences that could be mistaken for XMRV – a cautionary finding for researchers who have not yet produced and sequenced, as the WPI did, the entire virus. Recently Dr. Coffin, however, announced the creation of a test that could distinguish between ERV produced XMRV-like genetic sequences and XMRV produced sequences.
Dr. Coffin has noted that XMRV very closely resembles endogenous retroviruses found in inbred mice, which means lab mice, but that further research indicated it was ‘clearly distinct’ from them. In fact it would seem unreasonable, for XMRV not to resemble, at least in part, endogenous retroviruses since it appears to be, as do many species of MLV’s, partly derived from them (see below).
House Mice in the US are Clean! – Kozak points out that house mice in the US typically carry M/P ERV’s while endogenous retroviruses associated with XMLV’s are typically found in eastern Europe and Asia.
This is an interesting but not a critical point since she has identified X ERV’s in laboratory mice and house mice do not appear to be a concern regarding contamination. It’s interesting that the exception to this rule occurs in a population of mice found in Lake Casitas, Calif near Santa Barbara. Some of these ERV infected mice can produce infectious virus – once again demonstrating the possibility of picking up an MLV from a mouse that is not ‘infected’ with the virus but carries parts of the virus in its genome.
Infection with the Complete Virus
Those were endogenous retroviruses embedded in an organism’s genome – but what about live viruses? Are they able to sneak into and infect laboratory mice?
The Key Element: the Cellular Receptors – when you’re talking about who has what virus then you’re often talking about what ‘receptors’ an organism carries on it cells. Retroviruses sneak into cells by co-opting receptors on the surface of that cell. You or I or the mouse in our house are susceptible to a virus if we carry cells that that virus can unlock.
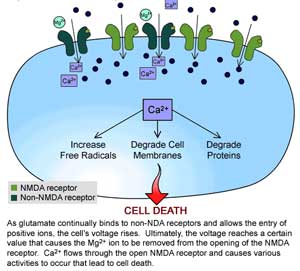 Cellular receptor XMLV’s and CFSMLVs are able to hijack a receptor on the surface of mouse cells called m-CAT while X and PMLVs can hijack the almost ubiquitous XPR1-1 receptor found in many animal species. It’s not clear whether XMRV entered humans through mice or another animal, but house mice have apparently been an important host in the spread of MLVs around the world to mammals, amphibians, even reptiles. Man, by providing suitable environments for house mice, has provided the vector for a retroviral attack across the world.
Cellular receptor XMLV’s and CFSMLVs are able to hijack a receptor on the surface of mouse cells called m-CAT while X and PMLVs can hijack the almost ubiquitous XPR1-1 receptor found in many animal species. It’s not clear whether XMRV entered humans through mice or another animal, but house mice have apparently been an important host in the spread of MLVs around the world to mammals, amphibians, even reptiles. Man, by providing suitable environments for house mice, has provided the vector for a retroviral attack across the world.
The Laboratory Mice – Since laboratory mice are probably the key figures in any contamination scenario the big question is whether or not their cells have receptors susceptible to XMRV. It turns out that most of them don’t. In fact, lab mice appear to be the only mouse group that carry a receptor that able to completely block X MLV (and therefore XMRV infection).
This is not completely surprising given that most laboratory mice are derived from three species of house mice (mus domesticus, castaneus and musculus)which differ in their susceptibility to XMLVs. Two of these species carry X -ERVs. Even though house mice from E. Europe and Asia area are more likely to carry XMRV permissive receptors, Kozak notes that house mice trapped at ‘various sites throughout Western Europe and the Americas’ do carry the receptor (Xpr1 svx) as well. But did that X-MLV receptor make its way into some lab mice?
- Mice and XMRV in CFS.
It turns out that it did. While not nearly as variable genetically as wild mice, laboratory mice did come from a variety of mouse populations some of which did carry the ‘wrong’ receptor. While the majority of lab mice do carry the ‘anti-XMRV receptor,’ some strains of lab mice carry a ‘permissive’ receptor that readily allows XMLVs to infect them. In fact Kozack recently penned an paper titled ‘Common inbred strains of Laboratory Mouse are Susceptible to…..Human Derived Retrovirus XMRV” which states that she found three inbred laboratory mice that could be infected by XMRV.
Overall the possibility of a contamination from a lab mouse appears to be lower for XMRV than for other MLVs, but the possibility is still present. This finding appears to be at odds with recent unpublished reports that no other mouse or other species have yet been found to harbor XMRV. Apparently the possibility of, or susceptibility to, infection does not necessarily denote the presence of infection.
Antibodies – the Critical Test – These findings demonstrate how vital finding antibodies are – something Dr. Mikovits has repeatedly pointed out. Since laboratory mice carrying ERVs or the actual virus could produce either gene sequences or the virus, simply finding the virus in a sample may not ultimately be enough, given the ubiquity of mouse DNA in the medical research and products line.
Antibodies tell a entirely different tale, though, because they are generated not by the virus or by viral-like elements in the person’s genome; but by the immune system itself in response to the virus. They are not an XMRV product – they are a human product and thus are one step removed from the actual virus.
That is presumably why so many groups are working on antibodies. One question about the antibody tests is whether they might possibly be reacting to a similar different virus. Dr. Bagni of the National Cancer Institute has reported produced an antibody test that is specific for XMRV. By May of this year, Dr. Singh had identified and found two antibodies for XMRV present in CFS patients. The WPI, of course, has their own improved antibody test. The NCI has elucidated a series of proteins in XMRV that can be used to produce antibody tests.
It Takes A Swarm?
We’ve talked about endogenous retroviruses and ‘real’ retroviruses separately – which is a mistake, because they are a) often found in the same cell and b) they often work together to produce new types of viruses.
The disease process associated with MLVs displays this family’s fascinating (and alarming) ability to merge and/or trade gene sequences with each other to create disease. Once ecotropic MLVs, for instance, enter a host they often recombine with gene products produced by M or X gene sequences embedded in the hosts genome to produce more virulent and dangerous viruses. The virulence of polytropic MLVs, on the other hand, can be enhanced by gene sequences produced by X-MRVs. The point is that this family loves family reunions; break one side of the family up and shove bits of it into your genome and it can still find a way to somehow re-unite with its long lost cousins. XMRV itself is a-product of two groups of MLVs intermingling.
(This brings up the question why these endogenous retroviruses are allowed to pump out foreign gene sequences that may be dangerous to the host? Are the dangerous sequences that transform a nasty virus into, say, a cancer causing one, more likely to be found in immune compromised or otherwise ill people (such as people with ME/CFS?)) Are some XMRV-positive people able to maintain their health because they are able to keep the other mouse DNA in their genome under control? These are intriguing questions. Dr. Huber scored first a CFIDS Association and then a full NIH grant to explore whether the HERV-K ERVs have come alive and are producing illness in people with ME/CFS. )
A Different Host – A Different Virus – A Fit with CFS? – That the XMRV found in humans is different from other XMLVs is not surprising given that viruses often become altered when they shift into another species. XMRVs appearance in humans coincided with a number of intriguing changes; one change appears to effect the sensitivity of the virus to interferons, an immune factor that has been connected indirectly with CFS via the RNase L pathway. Recently Dr. Mikovits announced that XMRV-positive people with ME/CFS had different interferon levels than XMRV-negative people – which seemed shocking at the time since altered interferon levels have never been associated with CFS – but could reflect the presence of XMRV.
XMRV also has a glucocorticoid response element – which suggests it is responsive to glucocorticoids such as cortisol which, of course, can be altered in ME/CFS.
A Very Active Group of Viruses
She writes in conclusion that
“the worldwide distribution of mice that carry MLVs and the broad host range of the X-MLVs suggest that we are only beginning to  describe what may be common and widespread interspecies transmissions… We do not know the evolutionary path taken by XMRV to humans, but multiple sequence and functional variations distinguish this virus from its MLV progenitors…The fact that all mice carrying infectious X-MLVs have one of three restrictive receptors suggests that unchecked X-MLV infection is likely to be deleterious…
describe what may be common and widespread interspecies transmissions… We do not know the evolutionary path taken by XMRV to humans, but multiple sequence and functional variations distinguish this virus from its MLV progenitors…The fact that all mice carrying infectious X-MLVs have one of three restrictive receptors suggests that unchecked X-MLV infection is likely to be deleterious…
